(2) Calculation of calorific value of fuel. The amount of heat released after each kilogram of liquid, solid fuel, or gas per cubic meter of gas is completely burned is called the calorific value of the fuel. From the list of thermodynamic functions: Since C has both amorphous carbon and graphitic carbon, the heat of formation of CO and CO 2 is somewhat different in some literatures. Therefore, the calorific value of C-containing gases such as CO, CH 4 and C 2 H 4 is different from the above table. . The air excess coefficient is too small, the combustion is incomplete, and the excessive air excess coefficient has an effect on the combustion temperature. The empirical air excess factor for various fuel combustions also varies with the combustion equipment. Generally, the gas fuel is selected from 1.02 to 1.2, the liquid fuel is 1.1 to 1.25, and the solid fuel is 1.3 to 1.7. Actual air demand: L n =nL 0
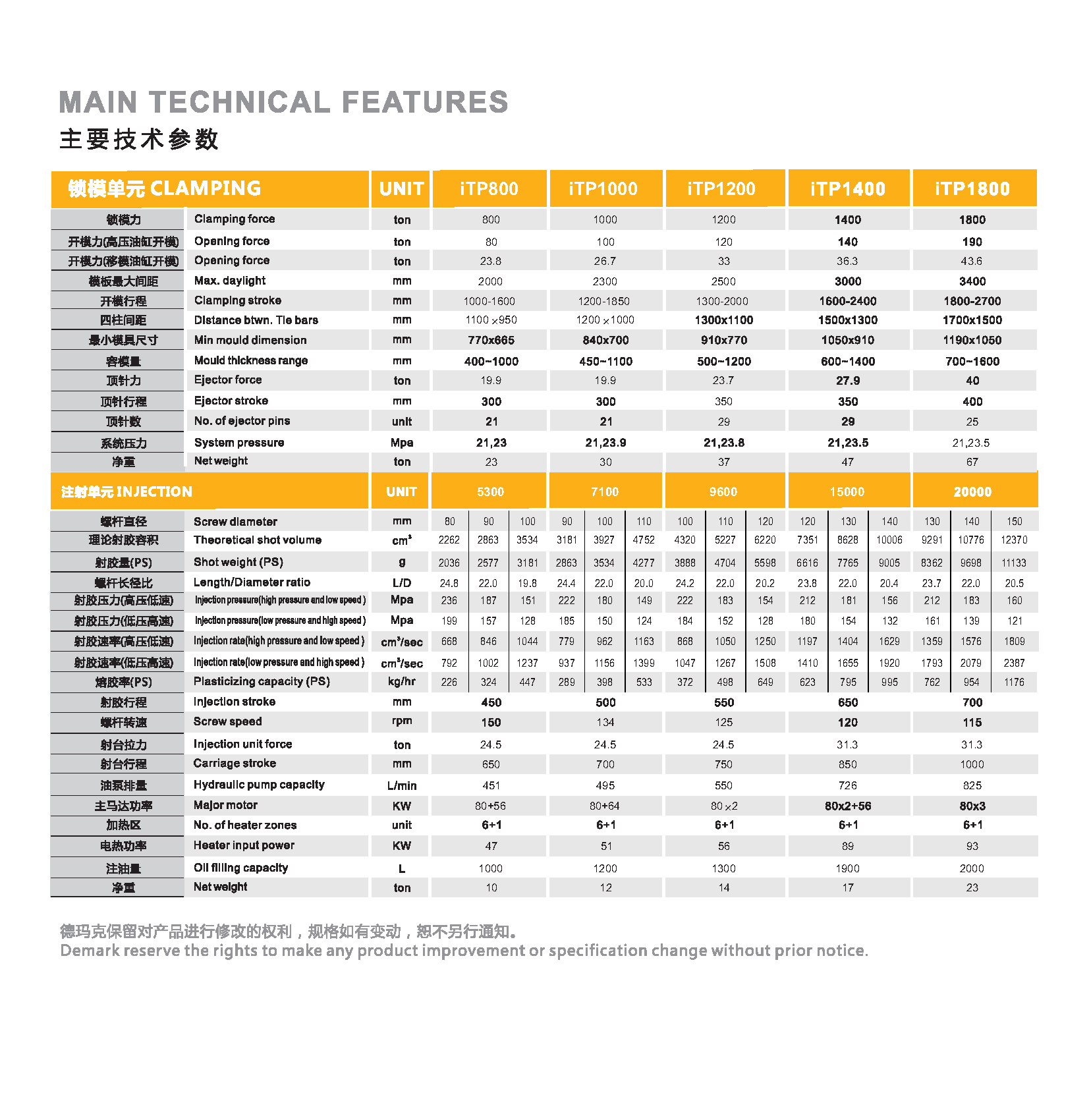
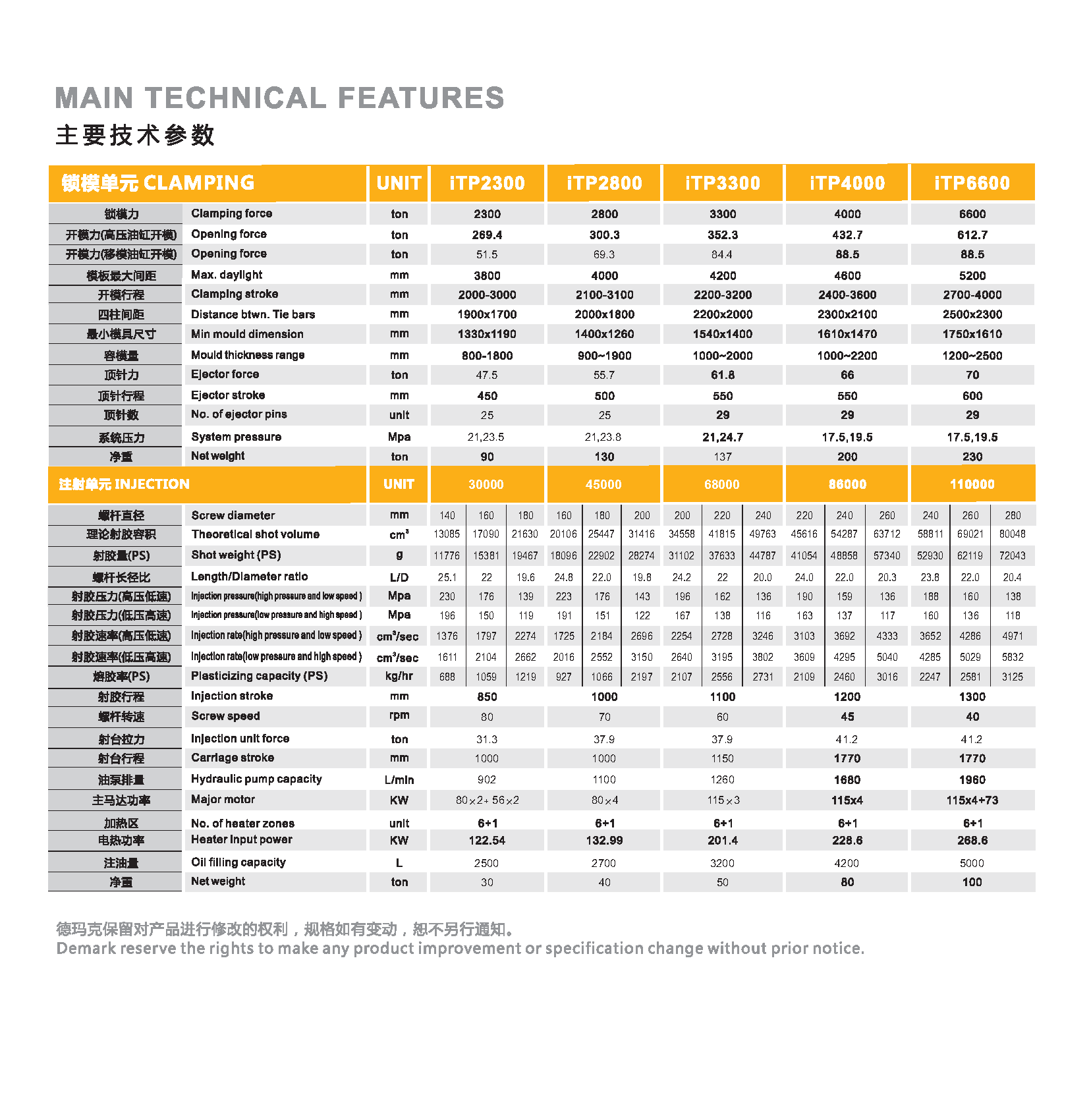
Servo Injection Moulding Machine,Hight Speed Injection Machine,Servo Energy Saving Injection Machine,Two Plate Machine Demark(changxing)injection system CO. LTD , https://www.petplas.com
The amount of heat released after complete combustion of the fuel is related to the state and temperature of the products of combustion. When the temperature of the combustion products is cooled to the original temperature of the reactants, and the water in the combustion products is in the state of 0 ° C water, the heat released by the complete combustion of the unit fuel is called the high calorific value of the fuel, expressed by the symbol Q high , and the unit is kilojoule / Meter 3 (gas) or kilojoules per kilogram (liquid or solid fuel). When the combustion product is cooled to the original temperature of the reactant, and the water in the combustion product is in a water gas state of 20 ° C, the heat released after the unit fuel is completely burned is referred to as the low calorific value of the fuel, which is represented by Q low , and the unit is the same as above. Most of the industry uses low calorific value to indicate the amount of heat generated by the fuel.
It is calculated from the thermodynamic function table ΔH 298 . For example, the combustion reaction of H 2 is: 
ΔH 298 H 2 O gas = -242.76 kJ / gram ΔH 298 H 2 O liquid = -287.28 kJ / gram Therefore, the thermal effect of H 2 when it is completely burned into moisture: 
The calorific value of the fuel can be determined experimentally or calculated by calculation.
1) the gas fuel of low calorific value calculation: After gas evolution complete combustion of the fuel shall be the sum of heat of combustible composition of coal gas of low thermal effect, namely:
Q = 12.7CO low wet wet + I0.8H 2 + 35.3CH 4 + 60.06C 2 H 4 wet wet wet + 23.18H 2 S (4)
If the absolute value of each wet component is substituted by the percentage specified in the calculation, the above formula becomes:
Q low = 0.127 CO wet + 0.108 H 2 wet + 0.355 CH 4 wet + 0.60 IC 2 H 4 wet + 0.2318 H 2 S wet (5)
Example: Find the low calorific value of the coke oven gas (the composition is the same as before).
Q low = 0.127 × 6.84 + 0.108 × 60.02 + 0.355 × 21.57 + 0.601 × 2.4
=0.8687+6.482+7.657+1.442=16.45mJ/ m3[next]
2) Low calorific value of liquid fuel (the same is true for solid fuel): The heat released after combustion of the liquid fuel includes the heat of decomposition of the organic compound and the heat of combustion of the combustible composition. Since the chemical structure of organic matter is unclear, the heat of decomposition is difficult to determine. Therefore, the empirical formula is used to calculate the calorific value of liquid and solid fuel. The commonly used empirical formula is:
With low Q = 33.89C + 102.93H with -10.878 (O use with -S) -2.5IW with (6)
In the calculation, if the supply components of each composition are substituted by the absolute value of the percentage, the above formula becomes:
With low Q = 0.3389C + 1.0293H with -0.1087 (O use with -S) -0.0251W with (7)
For example, the above-mentioned heavy oil supply component is known, and the low calorific value of the heavy oil is obtained.
Q low = 0.3389 × 85.6 + 1.0293 × 10.50 - 0.1087 (0.5 - 0.7) - 0.0251 × 2.0 = 39.87 million joules / kg 3) Determine the amount of air required for combustion and the volume of combustion products: all fuels contain a certain amount of flammable The amount of air required for a complete combustion reaction of a combustible in a fuel is called the theoretical air requirement for fuel combustion and is represented by the symbol L 0. The unit is m 3 /m 3 (gas fuel) or m 3 /kg ( Liquid and solid fuels. Since the combustibles in the fuel and the oxygen in the air require a certain amount of contact and reaction time before combustion, the theoretical air requirement is not sufficient to ensure complete combustion of the fuel. The amount of air added for this actual production is greater than the theoretical air requirement. This actual air demand is indicated by the symbol L n in meters 3 / m 3 or m 3 / kg. The ratio of the actual air demand to the theoretical air demand is called the air excess coefficient and is denoted by the symbol n. 
The volume of oxygen required to burn every 1 cubic meter of gas is: 
Excess air volume: L remaining = L n - L 0 = (n-1) L 0